Ved Prakash Roy, and Kevin J. Kubarych Chem. Phys. (2020) 531, 110653
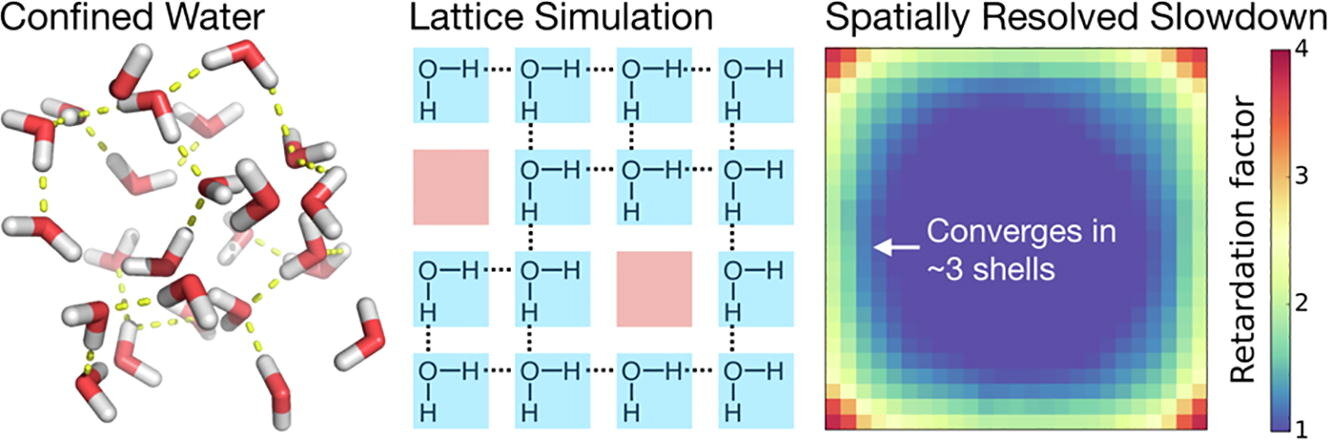
A finite, two-dimensional lattice model of liquid water captures the essential nearly four-coordinate hydrogen bonding network, while permitting a simple Metropolis Monte Carlo simulation in conditions ranging from crowded to dilute. This model examines excluded volume perturbations of hydrogen bond switching, avoiding complex topological and chemical heterogeneity of realistic interfaces. Retardation factors (relative to bulk) for switching agree with previous statistical models and atomistic molecular dynamics simulations of hydrated proteins. The model enables straightforward spatial mapping of retardation factors that are difficult to measure in atomistic simulations. The spatially-dependent retardation factors decrease exponentially from the interface. Simulating varying degrees of crowding, we do not find any cooperative, collective contributions anticipated from some recent spectroscopic observations suggesting correlated hydrogen bonding rearrangements of confined water. Longer-range cooperative interfacial influences may arise from complex chemical patterning of the surface, or to non-entropic influences such as multi-body interactions or altered hydrogen bond strengths.