Water is essential for life on Earth
Interfacial Hydration dynamics
The hydrophobic effect is essential for biological function since it is the main driving force for protein folding, membrane formation and interactions between biomolecules. Much is known about the thermodynamics (relative energies) associated with the hydrophobicity, but it is comparatively more challenging to probe the dynamics (i.e. motion, flexibility and fluctuations) experimentally due to the small number of hydrating water molecules relative to the vast amount of bulk solvent.
Using a novel protein labeling approach, where we covalently attach very strong IR probes site-specifically to the protein surface, we are able to sense both protein and water dynamics through their influence on the vibrational lifetime and spectral diffusion time scales of the probe chromophore.
This movie (below) is from our simulation studies of biomolecule hydration and highlights the heterogeneous nature of hydration water dynamics. Our group makes extensive use of atomistic simulations, analytical theory and simple models to develop chemically meaningful interpretations of our experimental data.
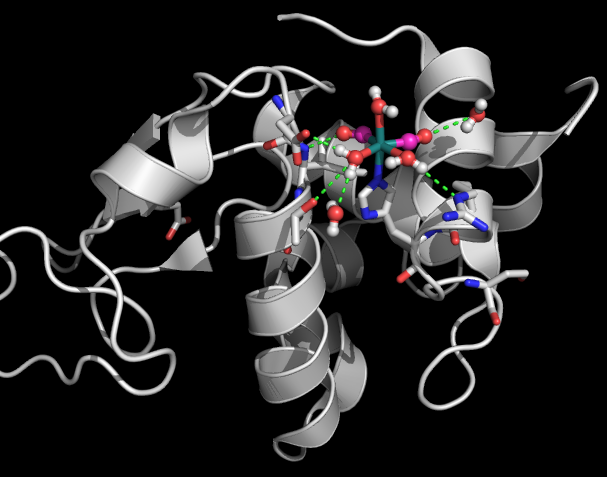
Site-Specific VIbrational Labeling
As we showed for the first time, transition metal carbonyl vibrations are remarkably sensitive to the presence of water. They are also able to tell us the nature of the water they sense: is the water bulk-like or confined? We found the influence of the protein on the water dynamics to be spatially heterogeneous.
Influence of Interfacial hydration dynamics on reactivity
It could be that the dynamical perturbation of interfacial water is simply a by-product of hydrophobic collapse, but it is also possible that there is a direct influence of this unique solvent on the chemical reactivity taking place within enzymes. We are building model, de novo metalloenzymes to test the link between hydration dynamics and long-range electron transfer in engineered systems.